Lab Scheduling with ORSOFT: The 360-degree view on your laboratory capacities and their holistic planning

Major obstacles and pitfalls of the traditional process approach
The conventional approach leads to a double-break of system discontinuity: On the process level, lab and production capacity planning is processed on different systems. For quality control, a laboratory information management system (LIMS) is often used, which is indeed is another, non-integrated process system. As a consequence,
- multiple systems with asynchronous data access may result in data breaks,
- providing lab planning with the data from production and the analytical laboratory may become subject to significant extra or additional costs, and
- often inaccurate and inefficient laboratory planning may occur as it cannot work with the most up-to-date production data.
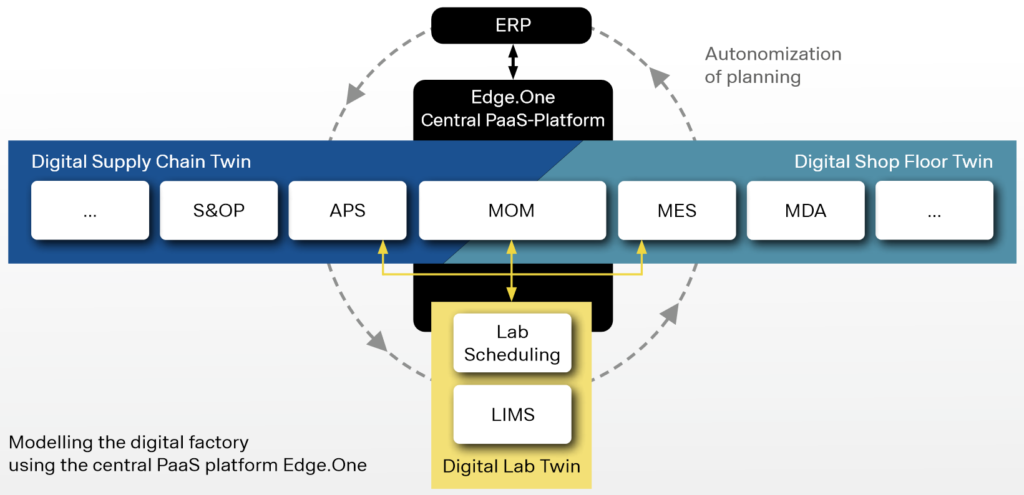
Modelling the digital lab twin: Simultaneous laboratory and production planning with ORSOFT LabScheduling
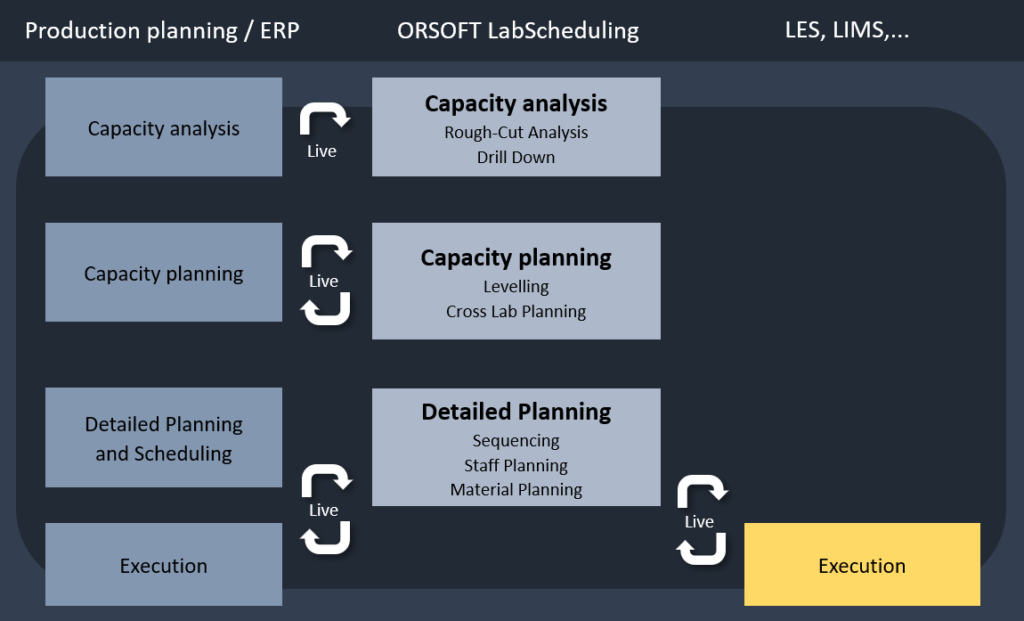
By using ORSOFT LabScheduling, laboratory planning is integrated into a company’s production planning and quality control process. Thus, the lab scheduling software obtains all relevant (real-time) data through an interface to the ERP system (certified SAP interface) and any LIMS, and stores the data in a common database.
A common data availability allows for booth, process-related integration and a temporal-planning alignment of lab scheduling with production planning. The planning of inspection lots (as a bottleneck within the production process) has thus the same planning horizon as the production planning itself and is additionally updated providing the most current information from the LIMS.
ORSOFT LabScheduling as innovative SAP application
In addition to the on-premise versions of LabScheduling, ORSOFT offers a “side-by-side extension” to a S/4HANA cloud system (SAP cloud platform). The extension can be used without on-site installation and is offered as a certified app in the SAP App Center.
As with the on-premise solution, the LabScheduling app allows to extend inspection lots from SAP QM with simulated ones generated by ORSOFT LabScheduling. This enables a planning-related consideration of future capacity requirements.